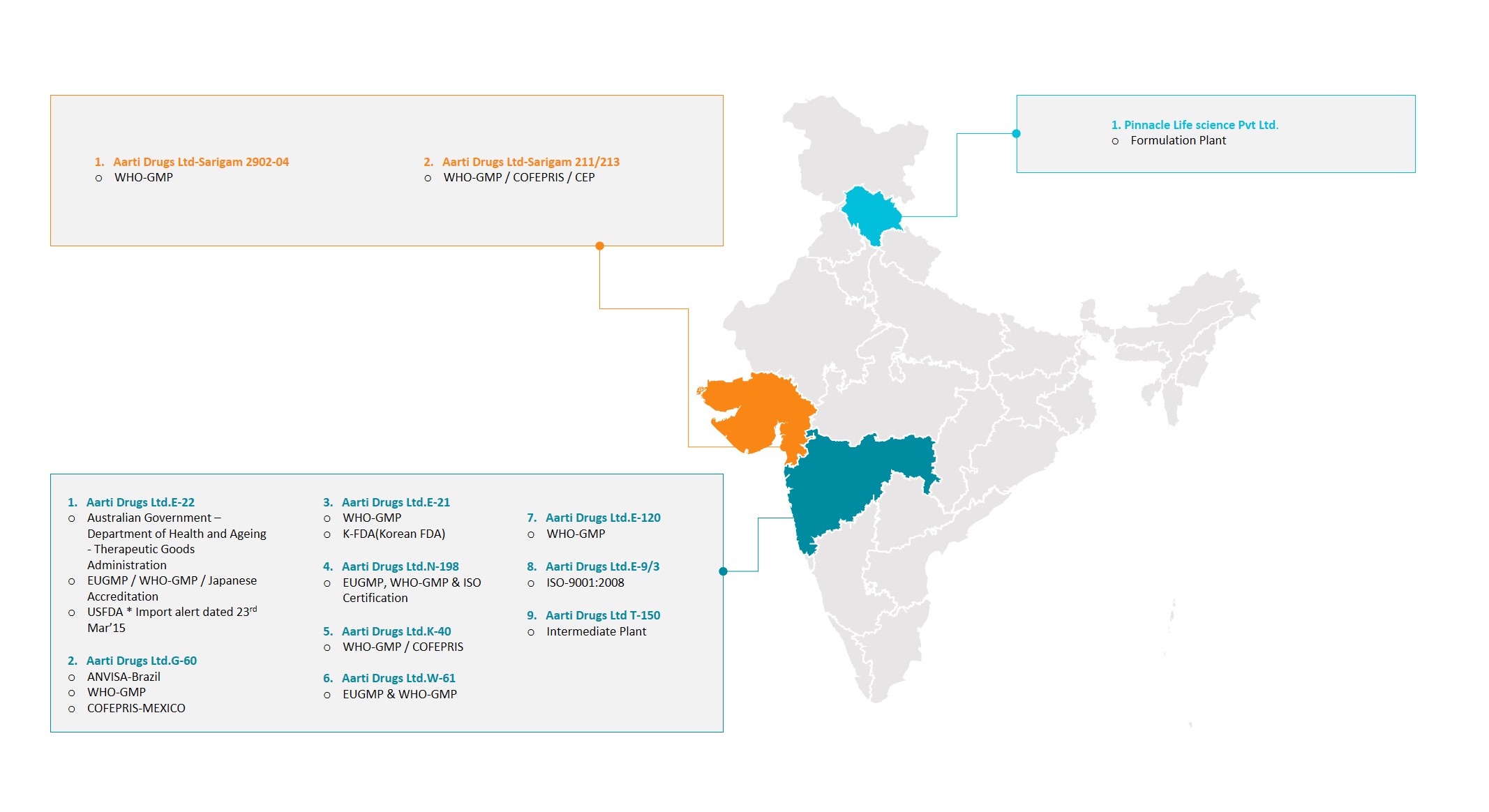
With two decades of manufacturing experience behind, the Company has today transformed into multi-ton, multi-location GMP complaint with the state-of the art facilities. Strategically located at Tarapur (Maharashtra) and Sarigam (Gujarat), the manufacturing units are currently capable of making over 50 bulk actives, several key intermediates and Speciality chemicals.
With (a strength of) eight manufacturing locations in its portfolio , Aarti Drugs Ltd. today, has total reaction capacity in excess of 1300 KL, consisting of SS and GL reactors across its units, varying from 0.5 KL – 18 KL.
Manufacturing in accordance with GMP regulations norms, Pilot Plants, Process development labs, In – Process QC labs and dedicated product processing areas are common features across every plant.
Unit processes for milling, sieving, blending, micronising and packing area are well in place. As a part of the pre-approval processes, these facilities have been inspected and approved by large end users, customers, who are rated amongst the largest companies in the world.
With the focus being to access potential US generics business, the Company has already commissioned and made operational, its new multipurpose manufacturing facility, strictly designed and built in accordance with USFDA requirements.
The Company’s core competence in manufacturing is due to its constant efforts to update processes, implement safety measures and focus on consistency in product quality.